![]() Nickerie.Net,
donderdag 18 november 2010
Nickerie.Net,
donderdag 18 november 2010
Nieuwe catheterbehandeling tegen hoge bloeddruk effectief
Eenmalige, effectieve behandeling te zijn tegen hoge bloeddruk mogelijk!
Submitted by Erasmus MC on 17 November, 2010
Onderzoeksresultaten van onder andere Erasmus MC gepresenteerd in VS Het terugdringen van hoge bloeddruk door middel van het gericht wegbranden van zenuwen rondom de nieren is effectief. Vandaag zijn op het jaarcongres van de American Heart Association de effecten van de nieuwe behandeling gepresenteerd. Onder andere Erasmus MC nam deel aan het onderzoek.
 Hoge
bloeddruk is wereldwijd de belangrijkste risicofactor voor vroegtijdig
overlijden. Bijna de helft van de Europeanen lijdt onder te hoge bloeddruk en
bij een deel van de mensen slaat een behandeling met medicijnen niet aan. Die
medicijnen moeten vaak levenslang geslikt worden en kunnen behoorlijke
bijwerkingen hebben. Wereldwijd worden de kosten voor gezondheidszorg die direct
gerelateerd zijn aan hoge bloeddruk geschat op 450 miljard Euro. Alhoewel de
meeste mensen wel weten dat hoge bloeddruk kan leiden tot een vroegtijdig hart-
of herseninfarct, weten weinig mensen dat de zenuwen rondom de nieren een
belangrijke rol spelen in de bloeddrukregulatie. Bij veel mensen met hoge
bloeddruk is dit mechanisme ontregeld.
Hoge
bloeddruk is wereldwijd de belangrijkste risicofactor voor vroegtijdig
overlijden. Bijna de helft van de Europeanen lijdt onder te hoge bloeddruk en
bij een deel van de mensen slaat een behandeling met medicijnen niet aan. Die
medicijnen moeten vaak levenslang geslikt worden en kunnen behoorlijke
bijwerkingen hebben. Wereldwijd worden de kosten voor gezondheidszorg die direct
gerelateerd zijn aan hoge bloeddruk geschat op 450 miljard Euro. Alhoewel de
meeste mensen wel weten dat hoge bloeddruk kan leiden tot een vroegtijdig hart-
of herseninfarct, weten weinig mensen dat de zenuwen rondom de nieren een
belangrijke rol spelen in de bloeddrukregulatie. Bij veel mensen met hoge
bloeddruk is dit mechanisme ontregeld.
De nieuwe catheterisatiebehandeling speelt hier op in door de zenuwen van binnen uit weg te branden. Dit houdt in dat een in de liesslagader ingebrachte catheter het zenuwweefsel door middel van radiogolven (radiofrequente ablatie) wegbrandt en uitschakelt. Daarmee worden zenuwsignalen geblokkeerd en het ontregelde mechanisme voor de bloeddrukregulatie gestopt.
Uit de resultaten van de studie die vandaag is gepresenteerd op het jaarcongres van de American Heart Association blijkt dat 6 maanden na de behandeling de bovendruk gemiddeld daalt met 32 mm kwikdruk en de onderdruk met 12 mm kwikdruk. Een dergelijke daling van de bloeddruk betekent meer dan een halvering van het risico om binnen 10 jaar aan hart- en vaatziekten te overlijden. Uiteindelijk heeft ongeveer 40% van de mensen na behandeling geen hoge bloeddruk meer.
Deze nieuwe behandeling tegen hoge bloeddruk is op de afdeling Radiologie in het Erasmus MC al bij meerdere patiënten met succes toegepast. Vandaag is de definitieve bevestiging gekomen dat de nieuwe behandeling ook echt werkt. Er lijkt dus een eenmalige, effectieve behandeling te zijn tegen hoge bloeddruk.
| Animatievideo van het systeem. |
Behandeling hoge bloeddruk met een Catheter Systeem from Nickerie.Net on Vimeo.
Ardian’s Catheter-Based Treatment for Hypertension Demonstrates Substantial and Sustained Blood Pressure Reduction in Landmark Randomized Clinical Trial
Published: 09:02 EST Wednesday the 17th of November 2010 | Press Release by Businesswire PDF RSS
Late-breaking data presented today at the American Heart Association Scientific Sessions 2010 and simultaneously published in The Lancet demonstrated that the landmark Symplicity HTN-2 trial evaluating Ardian’s Symplicity® Catheter System™ met its primary endpoint.
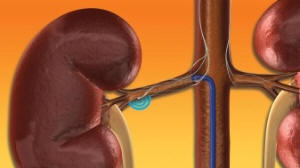 Once
in place within the renal artery, the Symplicity(R) Catheter System(TM) delivers
low-power RF energy to deactivate the surrounding renal sympathetic nerves.
(Photo: Business Wire)
Once
in place within the renal artery, the Symplicity(R) Catheter System(TM) delivers
low-power RF energy to deactivate the surrounding renal sympathetic nerves.
(Photo: Business Wire)
The study showed that, after six months, patients treated with Ardian's device experienced an average drop in blood pressure of 32/12 mmHg compared to an increase in blood pressure of 1/0 mmHg in the control group of patients treated with medical therapy alone (p<0.0001).
Research has shown that each incremental 20/10 mmHg increase of blood pressure above normal levels is associated with a doubling of cardiovascular mortality over a 10 year period1 and that reducing systolic blood pressure by as little as 5 mmHg can reduce the risk of stroke by almost 30 percent.2
“The impressive results of this study show that Ardian’s Symplicity System has the potential to become a truly revolutionary treatment,” said Murray Esler, M.D., Ph.D., principal investigator of the trial and associate director of the Baker IDI Heart and Diabetes Institute of Melbourne, Australia. “Combined with findings from the earlier Symplicity HTN-1 study, which demonstrated the safety and durability of the therapy out to two years, these results fuel our enthusiasm for the potential of this treatment to significantly impact the standard of care for the large number of patients suffering from this disease.”
The Symplicity HTN-2 trial was an international, multi-center, prospective, randomized, controlled study of the safety and effectiveness of renal denervation in patients with uncontrolled hypertension. One hundred-six patients were enrolled from 24 investigational sites. At baseline the randomized treatment and control patients had similar high blood pressures: 178/97 mmHg and 178/98 mmHg, respectively, despite both receiving an average daily regimen of five antihypertensive medications. After six months, the average blood pressure of the renal denervation group was reduced to 146/85 mmHg, compared to an average blood pressure of 179/98 mmHg for the control group.
The study also found that the therapy was safe, with no serious device or procedure-related events, no cardiovascular complications and no kidney-related complications.
“Hypertension is often described as a ‘silent killer’ as it frequently has no symptoms yet significantly increases a patient’s risk of heart attack, stroke or death,” said Andrew Cleeland, president and CEO of Ardian, Inc. “Positive data from the Symplicity HTN-2 trial reinforces our belief that this treatment has the potential to significantly improve the quality of life for the millions of people around the world with uncontrolled hypertension. We look forward to beginning a U.S. pivotal study of the device to gather data needed to bring this promising therapy to patients and physicians in the United States.”
About the Symplicity® Catheter System™
The Symplicity Catheter System is used to perform a procedure termed renal denervation (RDN). In a straight-forward endovascular procedure, similar to an angioplasty, the physician inserts the small, flexible Symplicity Catheter into the femoral artery in the upper thigh and threads it into the renal artery. Once in place within the renal artery, the device delivers low-power RF energy to deactivate the surrounding renal sympathetic nerves. This, in turn, reduces hyper-activation of the sympathetic nervous system, which is often the cause of chronic hypertension. The one-time procedure aims to permanently reduce blood pressure. RDN may also allow patients to reduce or eliminate the need for lifelong antihypertensive medications.
In addition to hypertension, the therapy may hold promise for treating heart failure, diabetes and chronic kidney disease, conditions also characterized by elevated sympathetic nerve activity. The Symplicity Catheter System has received CE Mark approval in the European Union and is investigational in the United States. Visit http://www.ardian.com/patients/symplicity.shtml to view an animation of the device and the procedure.
About Hypertension
Though it has no symptoms, hypertension (high blood pressure) is the number one risk factor for premature death worldwide, affecting about one in three adults.3 Nearly half of Europeans suffer from hypertension4 and in the United States, approximately 75 million people are affected, only two-thirds of whom are treated.5 Of those receiving treatment, approximately half are not achieving target blood pressure levels. The medications often prescribed for hypertension must be taken daily for the duration of a patient’s life, can be costly, and often result in side effects that can negatively impact quality of life. Globally, the estimated annual healthcare expenditure directly related to hypertension is approximately $500 billion.6
About Ardian
Privately held Ardian Inc., based in Mountain View, Calif., develops catheter-based therapies to treat hypertension and related conditions. Ardian is the eighth company created by The Foundry, a leading medical device incubator based in Menlo Park, Calif. Ardian’s investors include Morgenthaler Ventures, Advanced Technology Ventures, Split Rock Partners, Medtronic and Emergent Medical Partners. For more information, please visit www.ardian.com.
1 Lewington S., et al. Lancet. 2002;360:1903-1913.
2 Mohr, J.P., Churchill Livingstone, 2004.
3 Mathers, C., et al. World Health Organization; 2009.
4 Wolf-Maier, K., et al. JAMA 2003;289:2363-9.
5 Lloyd-Jones, D., et al. Circulation 2010;121;e46-e215.
6 Lawes, CM., et al. Lancet. 2008;371:1513-1518.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6515397&lang=en
| Bron / Copyright: | |
|
Nickerie.Net /Erasmus MC / Medical Facts |
17-11-2010 |
|
|
Email: info@nickerie.net
Copyright © 2010. All rights reserved.
Designed by Galactica's Graphics